Why Medicaid Programs Should Cover SMART for Asthma



Single Maintenance and Reliever Therapy (SMART) is a guideline-recommended, high-value asthma treatment that reduces preventable exacerbations and downstream health care utilization. SMART uses a single budesonide–formoterol inhaler for both maintenance and symptom relief, simplifying treatment and ensuring rapid delivery of anti-inflammatory therapy during symptom worsening.
Compared with traditional regimens, SMART reduces severe asthma exacerbations by approximately 30–40%, leading to fewer emergency department visits, hospitalizations, and systemic corticosteroid use.
Despite strong evidence and national guideline endorsement, SMART remains underutilized in Medicaid populations, largely due to formulary restrictions, non-preferred drug placement, and inhaler quantity limits. Improving coverage represents an immediate opportunity to improve outcomes and reduce avoidable costs. This policy brief summarizes the evidence for SMART and outlines actionable coverage recommendations for Medicaid programs.
Asthma facts

1 in 12 U.S. children have asthma.

~28 million Americans have asthma.

Asthma costs the U.S. more than $80 billion each year.
About SMART

Recommended by GINA and NAEPP guidelines.


~30–40% reduction in asthma attacks.
Recommended Medicaid Coverage Actions


Place budesonide–formoterol inhalers on preferred drug formulary (PDL).

Permit ≥2 budesonide–formoterol inhalers per month to enable both maintenance and reliever use.

Remove prior authorization requirement for SMART when guideline-indicated.
Why Asthma Matters for Medicaid
Asthma is a high-prevalence, high-cost chronic disease in the United States that drives substantial preventable health care utilization in Medicaid populations. More than 28 million Americans have asthma, and poor disease control leads to frequent exacerbations requiring acute care.¹
Why Traditional Asthma Therapy Falls Short


Historically, patients with asthma are prescribed:
A daily inhaled corticosteroid (ICS) controlled
A short-acting β-agonist (SABA) reliever (e.g., albuterol)
In practice, many patients underuse daily ICS therapy and over-rely on SABA rescue inhalers. This pattern is associated with higher exacerbation rates, increased emergency department visits, and avoidable hospitalizations.
These challenges are amplified in Medicaid populations, where ICS adherence is lower and SABA use is higher, contributing to disproportionate acute care utilization and costs.
To address this treatment gap, national and international guidelines recommend Single Maintenance and Reliever Therapy (SMART).⁵⁻⁶
SMART uses:
One inhaler combining an inhaled corticosteroid with rapid-onset formoterol for both daily maintenance and symptom relief.
By delivering anti-inflammatory therapy whenever symptoms occur, SMART improves adherence, reduces SABA overuse, and lowers exacerbation risk.

Everyday inhaler


Rescue inhaler

With SMART, everyday and rescue inhalers
are the same!
Traditional therapy: Daily ICS + separate rescue inhaler (two inhalers)
SMART: One ICS–formoterol inhaler for both maintenance and symptom relief (single inhaler)
SMART is recommended as preferred therapy by major national and international organizations:
The Global Initiative for Asthma (GINA)
U.S. Veterans Affairs / Department of Defense Clinical Practice Guidelines.
National Asthma Education and Prevention Program (NAEPP)
This broad consensus reflects strong evidence that SMART improves outcomes and reduces exacerbation risk compared with traditional therapy.
The Evidence for SMART
SMART Reduces Exacerbations and Acute Care Utilization
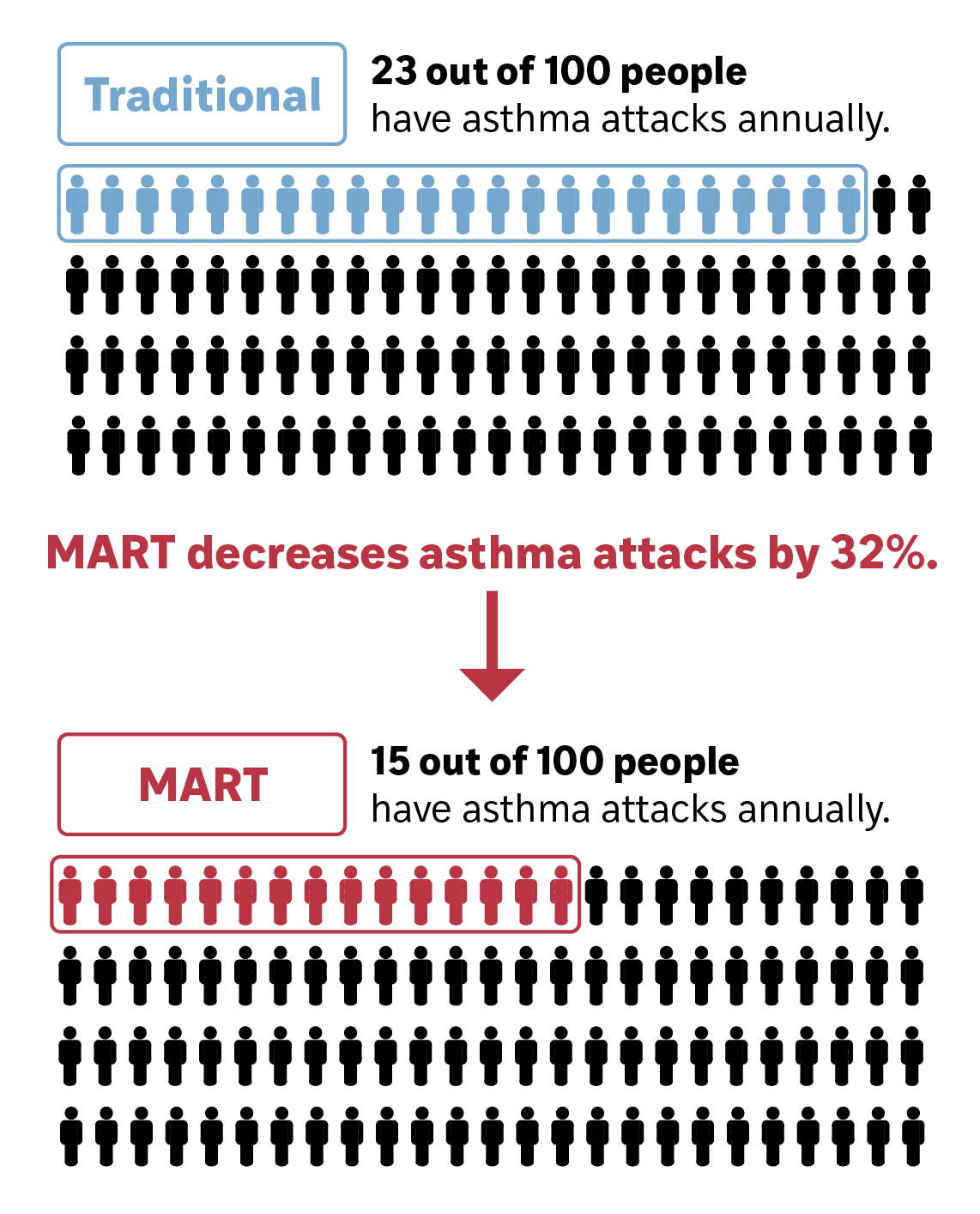
Randomized trials consistently show that SMART reduces severe asthma exacerbations and related health care utilization.⁷⁻⁹
Across randomized trials enrolling >22,000 patients, SMART reduces severe asthma exacerbations by ~30–40% compared with traditional maintenance plus SABA regimens.
Fewer exacerbations translate directly into fewer emergency department visits, hospitalizations, and systemic corticosteroid courses—major drivers of Medicaid spending.
SMART achieves these benefits without compromising symptom control while simplifying treatment with a single inhaler.
Patient Preference and Treatment Adherence
Patients consistently report that SMART is:
Easier to use
Simpler to remember
Better aligned with how they think inhalers should be used
Because patients use the same inhaler for symptoms and daily therapy, SMART improves adherence to anti-inflammatory treatment and reduces overreliance on rescue inhalers.

— 47-year-old Medicaid patient with asthma

SMART-Compatible Inhalers Supported by Guidelines
Two ICS–formoterol inhalers are clinically consistent with the SMART strategy:
Budesonide-formoterol (Symbicort®, Breyna®)
Mometasone-formoterol (Dulera®)
Budesonide–formoterol is the only inhaler explicitly recommended by both GINA and NAEPP for SMART, reflecting its use in most randomized clinical trials.
Evidence supporting mometasone–formoterol for SMART is limited and primarily extrapolated from the budesonide–formoterol evidence base.¹⁰
Implications for Medicaid Pharmacy Policy
Designate budesonide–formoterol as a preferred drug for SMART on Medicaid formularies.
Ensure coverage policies (quantity limits, prior authorization) support its use for both maintenance and reliever therapy.
Consider mometasone–formoterol as an alternative option where clinically appropriate.
GINA 2025 recommends ICS–formoterol–based therapy as preferred treatment for adolescents and adults with moderate-to-severe asthma. Source: Global Initiative for Asthma (GINA), 2025. Reproduced with permission. Available from www.ginasthma.org.
“SMART aligns anti-inflammatory treatment with real-world inhaler use, reducing preventable exacerbations and acute care events.”
— Anne Dixon, MA, BM, BC – Chair of Medicine; Director, Vermont Lung Center, University of Vermont School of Medicine
Addressing Policy Barriers to SMART Implementation
Despite strong evidence and national guideline support, SMART remains substantially underused due to coverage and formulary barriers.
In real-world practice, fewer than 15% of patients with moderate-to-severe asthma receive SMART, representing a major gap in guideline-concordant care.
Key Medicaid Pharmacy Policy Barriers
Non-preferred formulary placement: Budesonide–formoterol is often non-preferred, creating access barriers and limiting uptake of guideline-recommended therapy.
Lack of differentiation among ICS–LABA inhalers: SMART requires formoterol’s rapid onset of action, yet formularies frequently treat ICS–formoterol and ICS–LABA inhalers with slower-onset agents interchangeably.
Misaligned quantity limits: A single 120-actuation budesonide-formoterol inhaler may be insufficient for combined maintenance and reliever use, restricting appropriate SMART implementation.
These policy barriers reduce access to guideline-recommended therapy and contribute to preventable exacerbations and acute care utilization.

“When coverage policies align with how SMART is designed to be used, more patients receive guideline-recommended care and avoid hospitalizations.”

— Anna Volerman, MD, MPH, Professor of Medicine, Vermont Lung Center, pediatrician at the University of Chicago School of Medicine
Is Covering SMART Cost-Effective for U.S. Payers?
Yes. Evidence indicates that covering SMART is cost-saving or cost-neutral for U.S. health care payers, including Medicaid.
Although SMART may modestly increase pharmacy spending due to higher inhaler use, these costs are more than offset by reductions in asthma-related emergency department visits and hospitalizations–primary drivers of asthma spending.¹¹
SMART shifts costs from unplanned acute care to predictable pharmacy spending, improving budget stability while reducing total health care utilization.
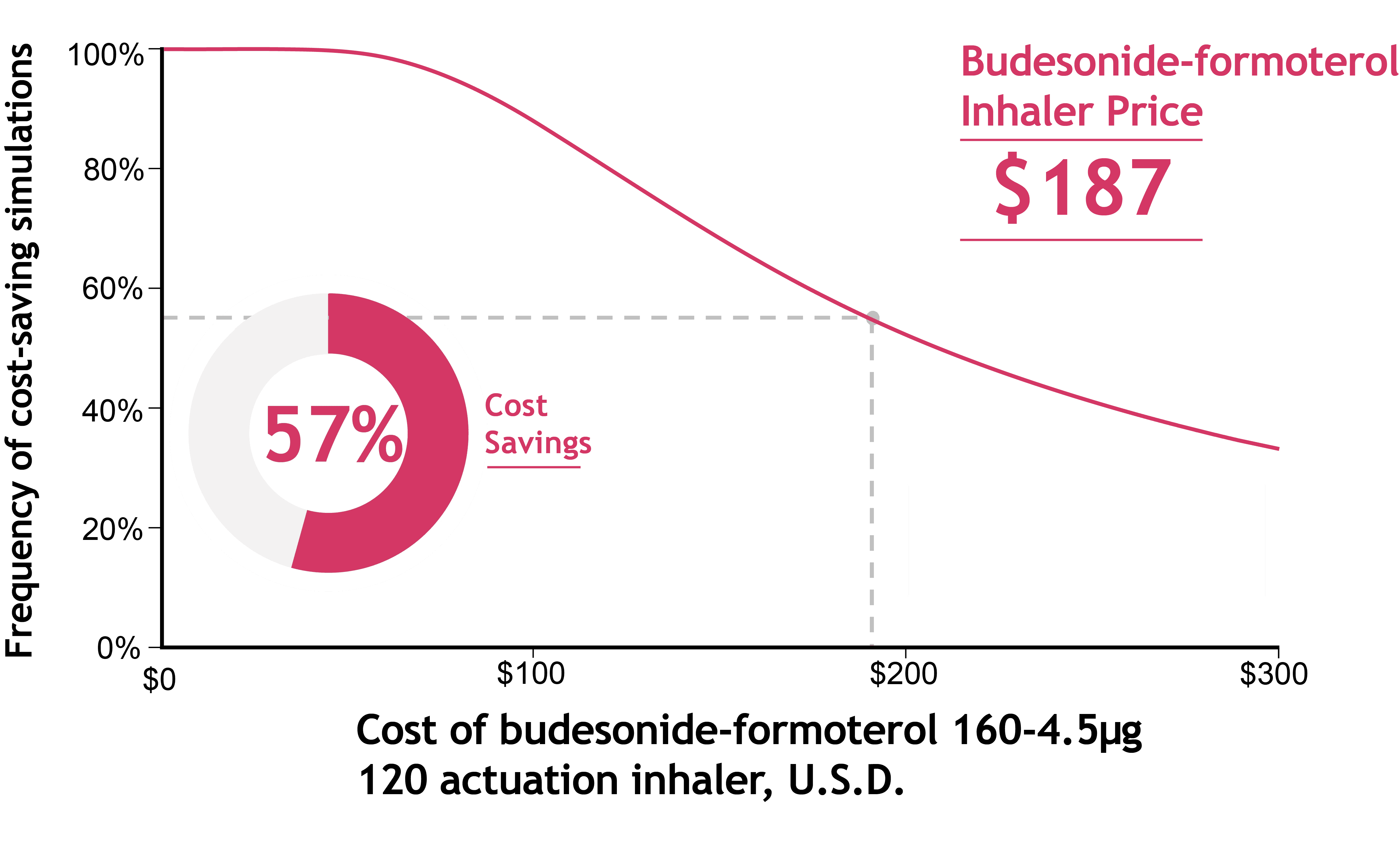
Budget Impact Findings
Economic modeling shows SMART is frequently cost-saving across a wide range of assumptions.
At an inhaler cost of $187, SMART was cost-saving in 57% of modeled scenarios, driven primarily by fewer emergency visits and hospitalizations.
Results remain favorable across varying exacerbation rates and inhaler prices.
Interactive Budget Impact Tool
Medicaid Coverage of SMART by State (2024)
State Medicaid programs vary widely in how they cover SMART therapy, including formulary status, prior authorization requirements, quantity limits, and patient cost-sharing.
These policy differences directly influence access to guideline-recommended therapy and asthma-related acute care utilization.
Key Coverage Dimensions Assessed
Preferred formulary placement (PDL status)
Prior authorization requirements
Quantity limits aligned with SMART dosing
Patient cost-sharing requirements
Recommended Medicaid Coverage Actions

Core Recommendations

Designate budesonide–formoterol as preferred on Medicaid formularies:
SMART requires formoterol’s rapid-onset of action.
Other ICS–LABA products are not appropriate substitutes for SMART.

Cover ≥2 budesonide–formoterol inhalers per month:
SMART requires the same inhaler for maintenance and relief.
Maintenance-only quantity limits restrict appropriate use.

Minimize or eliminate cost-sharing for SMART:
Higher inhaler costs reduce access and adherence.
Lower cost-sharing improves outcomes and reduces acute care utilization.
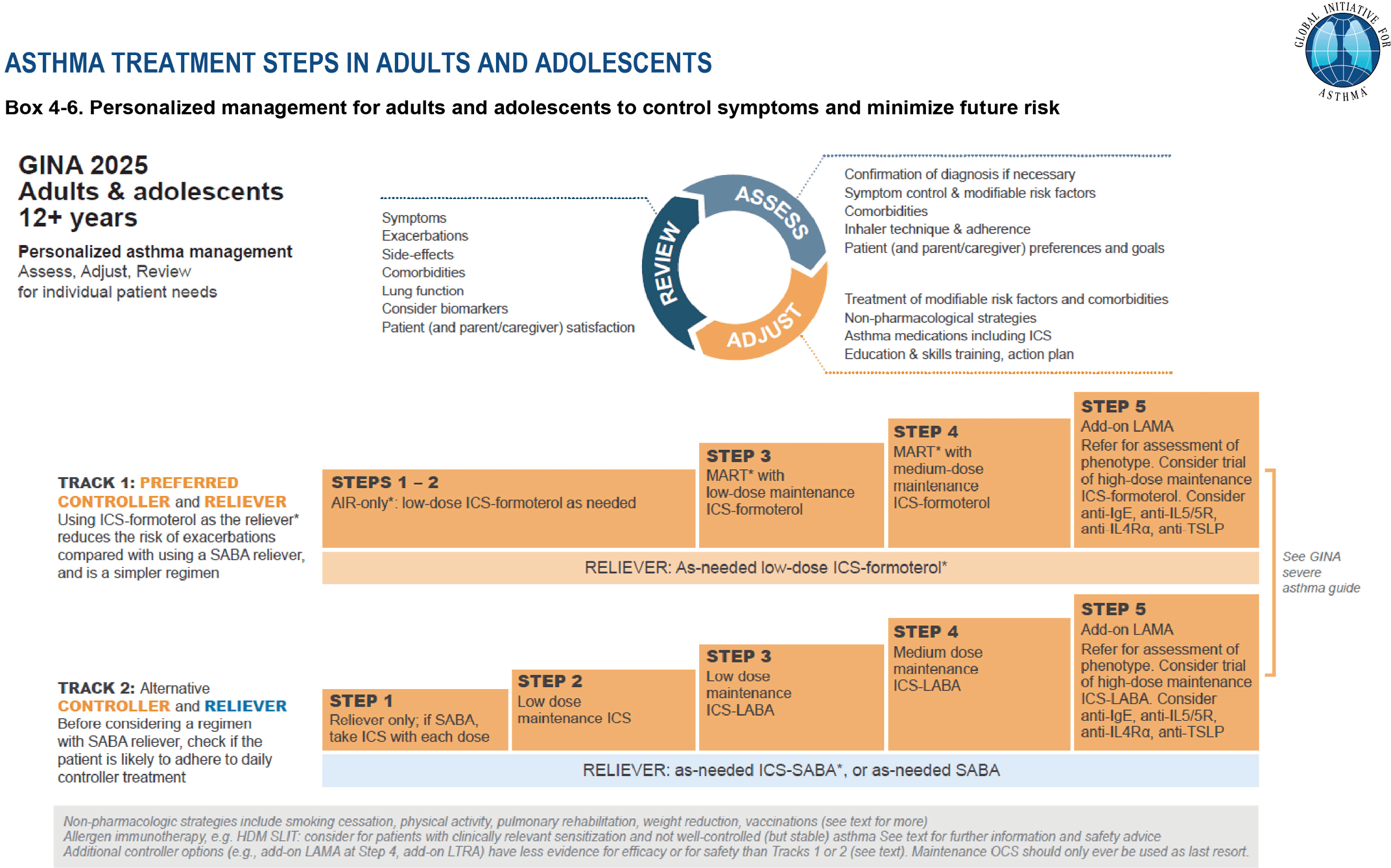
GINA 2025 SMART dosing recommendations
In the 2025 Global Strategy for Asthma Management and Prevention, GINA recommends SMART dosing based on age and asthma severity.
Because patients on Step 4–5 SMART therapy may use multiple inhalations daily, they can finish a 120-actuation inhaler before the end of the month. Also, both adults and children may benefit from a second inhaler stored separately for emergency use.
Therefore, we recommend that healthcare payors cover at least two budesonide-formoterol inhalers per month to support safe and effective SMART use.
SMART in children (Ages 6 to
11 years):
Inhaler: Budesonide-formoterol 80-4.5µg
Steps 1-2: no current evidence supporting use supporting SMART in this group.
Step 3: SMART: 1 inhalation once daily plus 1 inhalation as needed.
Step 4: SMART: 1 inhalation twice daily plus 1 inhalation as needed.
Step 5: SMART: no current evidence supporting use supporting SMART in this group.
SMART in adolescents & adults (Ages 12 and up):
Inhaler: Budesonide-formoterol 160-4.5µg
Steps 1–2 (anti-inflammatory reliever therapy [AIR]): 1 inhalation as needed.
Step 3: SMART: 1 inhalation once or twice daily plus 1 inhalation as needed.
Step 4: SMART: 2 inhalations twice daily plus 1 inhalation as needed.
Step 5: SMART: 2 inhalations twice daily plus 1 inhalation as needed.
Case Example: Missouri Medicaid (MO HealthNet)
Missouri Medicaid (MO HealthNet) expanded SMART access by aligning pharmacy policy with national asthma guidelines to increase controller use and reduce preventable acute care utilization.
Key Policy Actions Taken by MOHealthNet:
Designated budesonide–formoterol (and mometasone–formoterol) preferred therapies without prior authorization.
Allowed up to three inhalers per month to support SMART dosing.
Limited adult SABA coverage to three inhalers every six months without prior authorization
These policies illustrate how Medicaid programs can operationalize SMART coverage.
“Coverage policies that support SMART make it easier for clinicians to deliver guideline-based care and for patients to use their medications as intended.”
— Joshua Moore, MO HealthNet Director
References
Contact:
James G. Krings, MD, MSc
Washington University in St. Louis
kringsj@wustl.edu
Krutika Chauhan, MBBS, MPH, CPH
Washington University in St. Louis
c.krutika@wustl.edu
“I like the combined approach [SMART] because I forget to take my daily inhaler, but I don't forget to take my rescue inhaler because my body tells me when I need it."
— 47-year-old patient with asthma



Funding and Acknowledgments
This policy brief was supported by a Public Health Policy grant from the American Lung Association (ALA). The views expressed are those of the authors and do not necessarily reflect the views of the ALA.
We thank Ted Floros, Adjunct Professor at Washington University in St. Louis, and his students Biruk Denma and Kaleb Urga for their contributions to data visualizations.